Significance of Pitting Resistance Equivalent Number (PREN) in Tubing Material Selection
Significance of Pitting Resistance Equivalent Number (PREN) in Tubing Material Selection
Common in high-chloride environments at elevated temperatures, pitting corrosion causes small cavities, or pits, to form on the surface of a material. It begins when the passive oxide layer on the metal’s surface breaks down, making the metal susceptible to the loss of electrons. When an electron from the metal escapes, iron in the metal dissolves into a solution in the bottom of the pit, diffuses toward the top and ultimately oxidises to rust. As the pit gets deeper, the iron chloride solution concentration in the pit can increase and become more acidic, which in turn accelerates the pit growth. Eventually, the corrosion may lead to perforation of tubing walls and leaks.
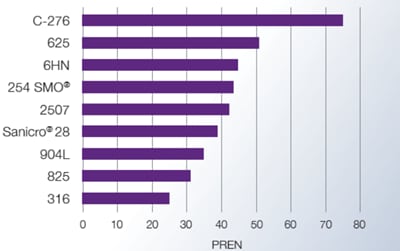
A parameter for comparing the resistance to pitting in chloride environments is the PRE number (Pitting Resistance Equivalent). Pitting Resistance Equivalent Number or PREN value is calculated
PREN = %Cr + 3.3 x (%Mo + 0.5W) + 16 x %N
Higher PREN values indicate greater pitting corrosion resistance.
Pitting corrosion is best prevented by proper alloy selection. Different tubing metals and alloys can be compared using their Pitting Resistance Equivalence Number (PREN), which is calculated from the chemical composition of the material. PREN increases with higher levels of chromium, molybdenum, and nitrogen in the alloy.
Need more Information or looking for a specific product?
Just fill in the below form and we will come back at the earliest